Stroke Quality Metrics

Stroke Quality Metrics
Our stroke volume and outcomes data is publicly reported according to Comprehensive Stroke Center requirements.
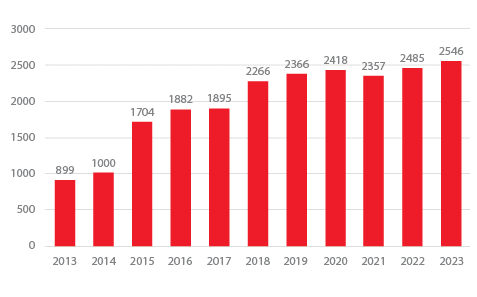
Stroke Volumes
Grady has one of the highest stroke and treatment volumes in the country, treating over 2546 patients in 2023, with the volume continuing to increase.
Stroke Treatment Rates
In 2023, Grady Hospital switched from the intravenous (IV) clot-busting drug Activase to its now preferred Tenecteplase, and administered this drug in 12.6% of stroke cases – above the regional and national average. Our team also performed endovascular treatment (clot retrieval) in 27.2% of stroke cases – almost four times the regional and national average.
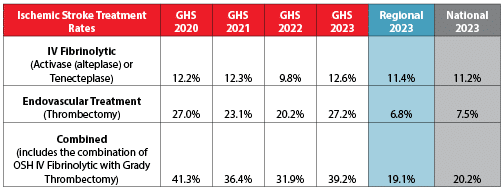
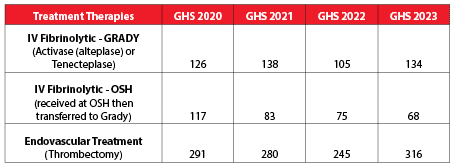
Ischemic Stroke Treatment Volumes
This shows the number of patients who not only received the IV clot-busting drug Activase, or Tenecteplase (replaced Activase in July 2023), at Grady Hospital, but who also had endovascular clot retrieval at Grady or Activase at an outside hospital before being transferred to Grady for a higher level of care.
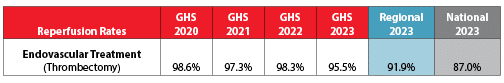
Ischemic Stroke Reperfusion
Reperfusion means restoring circulation to a part of the brain that was blocked by a blood clot in the artery. Grady stroke experts opened blocked arteries using endovascular thrombectomy 95.5% of the time! That is above both average regional and national rates.
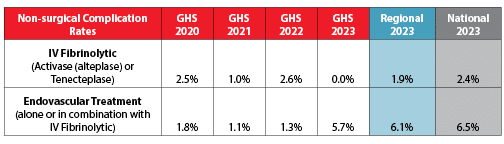
Nonsurgical Complication Rates
Grady not only treats more strokes, but our patients experience fewer complications, with rates below the average regional and national averages.
Source: American Heart Association /American Stroke Association Get with the Guidelines data, January 2017-December 2023
Nationally Recognized Care

In 2023, Grady Hospital achieved the highest recognition for quality and speed, the American Heart Association/American Stroke Association, Get With The Guidelines® ‒ Stroke Gold Plus, Target Stroke Honor Roll Elite Plus. This award recognizes facilities that maintain an 85% or higher performance rate for quality indicators (measures used to gage stroke care performance) for two or more years, and achieve arrival to Activase administration times of ≤ 60 minutes in 75% and within 45 minutes in 50% of applicable acute ischemic stroke patients.